Johnson & Johnson's COVID-19 vaccine trial pause explained by experts, history
On Monday afternoon, Johnson & Johnson announced that it is pausing its clinical trials for the company’s COVID-19 vaccine candidate after a study participant became ill. Details are scarce, but Johnson & Johnson said in a press release that the patient had an “unexplained illness.”
“Adverse events — illnesses, accidents, etc., even those that are serious, are an expected part of any clinical study, especially large studies,” the company said in the press release, adding that there will be a “careful review of all of the medical information before deciding whether to restart the study.”
The news comes just a month after drugmaker AstraZeneca announced that it was pausing its COVID-19 vaccine trial due to an unspecified illness in a study participant. The company’s CEO later reportedly said in a phone call with investors that the patient was experiencing symptoms related to a rare neurological disorder.
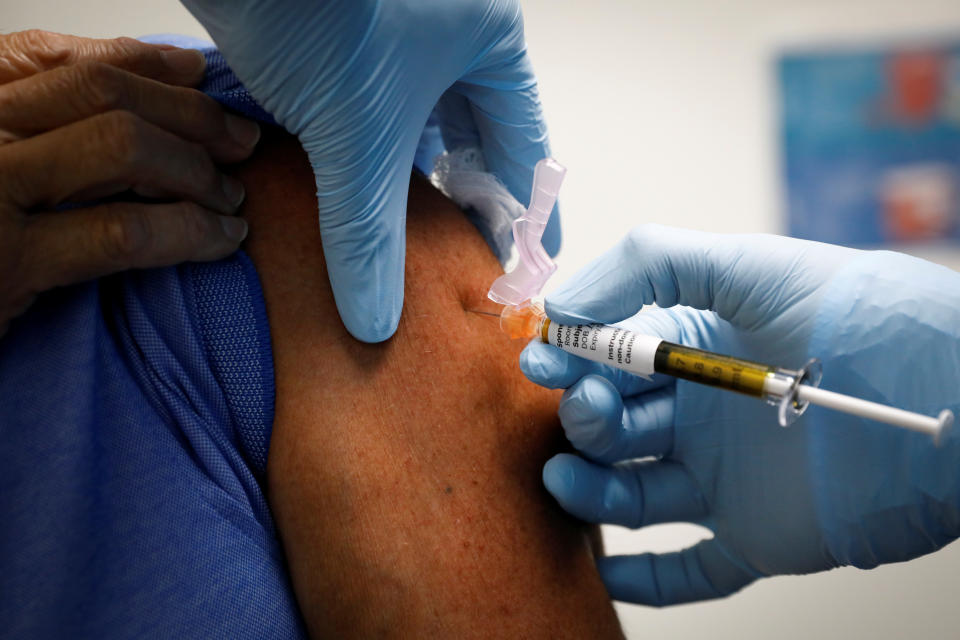
While the incidents do little to squash growing concerns over the rushed timeline of the COVID-19 vaccine, experts say these things can happen. “All vaccines can have side effects, and manufacturers have to balance safety and efficacy carefully,” Dr. Richard Watkins, an infectious disease physician in Akron, Ohio, and a professor of internal medicine at Northeast Ohio Medical University, tells Yahoo Life. “Most vaccine trials have temporary stops, so this is not unexpected.”
There are concerns, however, about rushing a vaccine. “When vaccines are developed, usually there is a process where we watch people in phase III for years to look for serious side effects,” Dr. Amesh A. Adalja, senior scholar at the Johns Hopkins Center for Health Security, tells Yahoo Life. “If you’re going to release vaccines before that time frame, there’s always a chance that a late side effect may turn up.”
There’s also this to consider, Adalja says: “There’s a chance too that when you move from vaccinating tens of thousands of people to millions, a side effect will become substantial.”
Americans have publicly expressed concern that the vaccine trials for COVID-19 are moving at too fast a pace. But Dr. Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, tried to calm those fears when he testified in front of a House subcommittee this past summer, stressing that government agencies would do due diligence before a vaccine would be released to the public.
Still, there have been times in the past where vaccines have been rushed or the timing has been off. These are a few incidents to be aware of.
1955: The Cutter incident
In 1955, some batches of the first vaccine to protect children against polio, which were produced by Cutter Laboratories, accidentally contained the live polio virus. As a result, it caused an outbreak of 250 cases of the disease, the Centers for Disease Control and Prevention says. The Cutter Incident led to “many cases of paralysis,” the CDC says, and the vaccine was recalled.
“This was a result of Cutter Labs not being able to deactivate the virus,” Adalja says. “The manufacturing process was flawed at that specific lab.” The CDC now says online that this was a “defining moment” in the history of vaccine manufacturing and government oversight of vaccines.
“The only good that came out of that awful episode was that the FDA ramped up its resources and became aware that they had to regulate not only the kind of vaccine, but how it is manufactured,” Dr. William Schaffner, an infectious disease specialist and professor at the Vanderbilt University School of Medicine, tells Yahoo Life.
After the process was improved, polio vaccinations resumed.
1976: Swine flu
In 1976, federal health officials halted vaccinations for swine flu after some recipients developed a serious neurological disorder known as Guillain-Barre Syndrome (GBS). According to the CDC, the risk was approximately one additional case of GBS per 100,000 people.
The incident was later investigated by the Institute of Medicine, which found that people who received the 1976 swine influenza vaccine had an increased risk for developing GBS. It’s still unclear why this happened, though.
Schaffner says this is “still being debated today,” given how small the increased risk of GBS was to patients. “Guillain-Barre can also be caused by the influenza virus itself,” he says. “You always need in these incidents of extremely rare adverse events to measure protection you get against an increased risk of adverse side effects.”
1998: Rotavirus and intussusception
In 1998, the FDA approved the RotaShield vaccine, the first to prevent rotavirus. But, soon after it was licensed, some infants who received the vaccine developed intussusception, a rare type of bowel obstruction that happens when the bowel folds in on itself, the CDC says. “There was not a signal about intussusception in the vaccine trials — it happened at such a low frequency that the clinical trials did not pick it up,” Schaffner explains. “But when it started to be used in large numbers, the surveillance system started to pick up cases.”
The CDC eventually halted the use of the vaccine. “It was succeeded with a safer rotavirus vaccine,” Schaffner says.
Adalja urges the general public — and vaccine developers — to have patience with the vaccine development process. “This is usually something that takes years,” he says. “They’re trying to streamline processes that need to be streamlined.”
But Adalja says that, while there is an “urgency to get this out,” it comes with drawbacks. “We will have some safety data, but not complete safety data,” he says. “There is going to be some risk benefit. Hopefully, everyone will be transparent and not politicize this so that people can make these decisions with their doctor.”
For the latest coronavirus news and updates, follow along at https://news.yahoo.com/coronavirus. According to experts, people over 60 and those who are immunocompromised continue to be the most at risk. If you have questions, please reference the CDC’s and WHO’s resource guides.
How to maintain your physical and mental health during the pandemic
Taking care of a loved one with COVID-19? Here’s how to stay healthy
Q&A with Dr. Kavita Patel: How to keep your family safe and maintain your mental health
Read more from Yahoo Life
Want lifestyle and wellness news delivered to your inbox? Sign up here for Yahoo Life’s newsletter.
 Yahoo Sports
Yahoo Sports 
