AstraZeneca halts COVID-19 vaccine trial after 'unexplained illness'
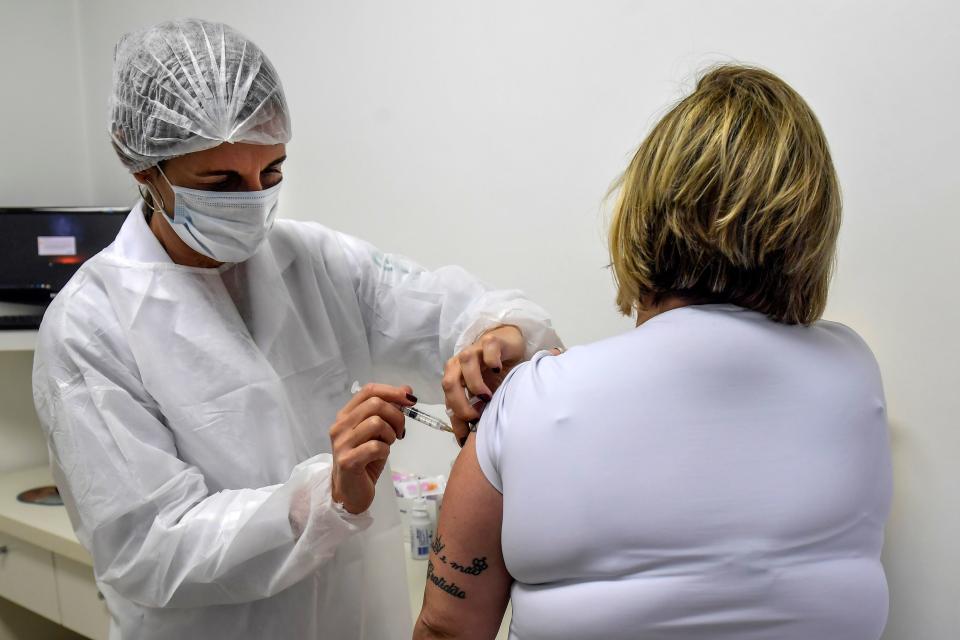
AstraZeneca (AZN.L) has paused human trials of a COVID-19 vaccine developed in partnership with the University of Oxford.
StatNews, a specialist medical website in the US, first reported on Tuesday evening that AstraZeneca had paused a large Phase Three trial of AZD1222, an experimental vaccine previously known as ChAdOx1 nCoV-19.
A spokesperson for AstraZeneca confirmed to Yahoo Finance UK the trial had been halted after the discovery of an “unexplained illness” in one patient.
Video: Vaccine trial paused after a participant's illness
A spokesperson said the pause was voluntary and part of a “standard review process.” An independent committee will now review the incident and decide whether to resume.
“This is a routine action which has to happen whenever there is a potentially unexplained illness in one of the trials, while it is investigated, ensuring we maintain the integrity of the trials,” the AstraZeneca spokesperson said.
“In large trials, illnesses will happen by chance but must be independently reviewed to check this carefully.”
The independent review could take as little as a few days. If the illness is found to be unconnected to the vaccine, the trials will be restarted.
“At AstraZeneca we put science, safety and the interests of society at the heart of our work,” Pascal Soriot, chief executive of AstraZeneca, said in a statement. “This temporary pause is living proof that we follow those principles while a single event at one of our trial sites is assessed by a committee of independent experts.”
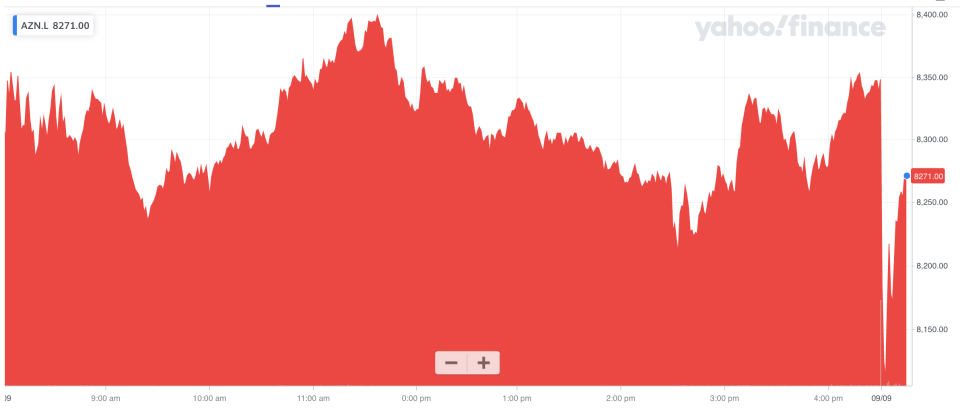
Shares in AstraZeneca fell over 2.5% in early trade in London, before paring back some losses.
The AZD1222 vaccine has been developed in conjunction with the University of Oxford and is one of the most closely-watched potential COVID-19 vaccines in development around the world. Europe had already signed a deal with AstraZeneca to produce 400 million doses of the vaccine in anticipation of successful trials, while the US had ordered 300 million doses.
“The Oxford vaccine in many ways is right at the front because it’s been into more people than anything else,” Sir Patrick Vallance, the UK’s chief scientific advisor, said during a press conference on Wednesday.
30,000 people across Brazil, the UK, US, and South Africa are currently taking part in trials of the drug.
AstraZeneca’s spokesperson didn’t give details about what specifically triggered the pause. StatNews reported that it was triggered by “a suspected serious adverse reaction in a participant in the United Kingdom.”
READ MORE: AstraZeneca launches new coronavirus prevention and treatment trial
Dr. Adam Barker and Dr. Tara Raveendran, healthcare analysts at stockbroker Shore Capital, cited reports suggesting the adverse affect could be transverse myelitis (TM), which is an inflammation of the spinal cord.
Experts said more details were needed to draw any conclusions from the pause.
“Adverse events aren’t uncommon in clinical studies and it could certainly be nothing to do with the vaccine (most aren’t), but the very sensible (if not only) thing to do in such a circumstance is to temporarily suspend the study and investigate what has happened,” Barker and Raveendran wrote in a note on Wednesday morning.
Vallance said pauses to Phase Three trials were “not an unusual thing” and were “sensible.”
“That’s precisely why Phase Three clinical trials happen,” Vallance said. “We need to make sure with these vaccines that they work, they work well enough, and they are safe.”
The AZD1222 trial had been due to conclude in November but Barker and Raveendran said this pause would likely delay that timeline.
READ MORE: AstraZeneca and University of Oxford team up on potential COVID-19 vaccine
The spokesperson for AstraZeneca said: “We are working to expedite the review of the single event to minimise any potential impact on the trial timeline. We are committed to the safety of our participants and the highest standards of conduct in our trials.”
Soriot said the company would be “guided by [the independent review] committee as to when the trials could restart.”
 Yahoo Sports
Yahoo Sports 