Three positive things we learned about coronavirus drug treatments today
Since the beginning of the coronavirus outbreak that has claimed the lives of more than 600,000 people worldwide, scientists around the globe have been racing to create an effective vaccine.
On Monday, the public has been given the first glimmer of hope that there could soon be a treatment to slow the pandemic and lessen the disease’s damage.
As it stands, only there is only one drug licensed in the UK to treat coronavirus.
Dexamethasone, an anti-inflammatory drug funded by the UK government, has been approved to treat all UK hospitalised COVID-19 patients requiring oxygen, including those on ventilators.

The drug has been proven to reduce the risk of death significantly in COVID-19 patients on ventilation by as much as 35% and patients on oxygen by 20%, reducing the total 28-day mortality rate by 17%.
The World Health Organization (WHO) says: “At this time, there are no specific vaccines or treatments for COVID-19. However, there are many ongoing clinical trials evaluating potential treatments.”

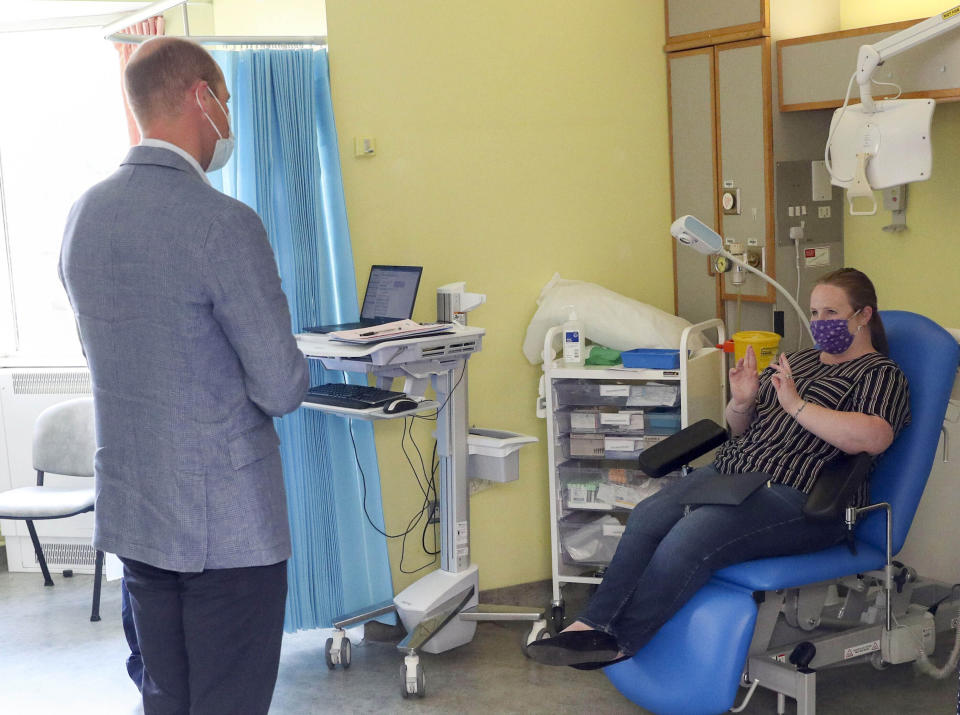
Oxford University trial finds vaccine ‘safe’
A coronavirus vaccine developed by Oxford University appears to be safe and induces a “strong” immune reaction.
The results of tests carried out by the university were published on Monday in The Lancet medical journal.
The early stage trial, which had 1,077 participants aged between 18 and 55, found that the vaccine is safe and causes few side effects.
The vaccine – called ChAdOx1 nCoV-19 – also induces strong immune responses in both parts of the immune system, provoking a T cell response within 14 days of vaccination, and an antibody response within 28 days.
Read more: 'Broken heart syndrome' on the rise during coronavirus pandemic
Compared with the control group of those given a meningitis vaccine, the COVID-19 vaccine caused minor side effects, which were treatable with paracetamol.
However, The Lancet warned of the trial: “Current results focus on immune response measured in the laboratory. Further testing is needed to confirm if vaccine effectively protects against infection.”
We’ve secured early access to 90 million doses of promising coronavirus vaccine candidates from @BioNTech_Group, @pfizer and @valnevaSE, and treatments containing neutralising antibodies from @AstraZeneca to protect those who cannot receive vaccines.
https://t.co/TGBcLpqPzg pic.twitter.com/Ev609t4Bl8— UK Prime Minister (@10DowningStreet) July 20, 2020
Boris Johnson said it was an “important step in the right direction”.
"This is very positive news. A huge well done to our brilliant, world-leading scientists & researchers at @UniofOxford," the prime minister said on Twitter.
The university’s Professor Sarah Gilbert, co-author of the study, said: “There is still much work to be done before we can confirm if our vaccine will help manage the COVID-19 pandemic, but these early results hold promise.”
The journal said: “Authors say further clinical studies, including in older adults, should be done with this vaccine.”
UK secures 90 million doses of ‘promising’ coronavirus vaccines
The government has secured early access to 90 million doses of “promising” coronavirus vaccines currently being developed.
The agreements include 30 million doses of a vaccine in trials that are being developed by BioNTech and Pfizer and 60 million doses of a vaccine from Valneva.
These will add to the 100 million doses of the vaccine under development by the University of Oxford and AstraZeneca that has been found to “induce a strong reaction” and is already in large-scale phase 3 human trials.
Read more: What is the coronavirus treatment being hailed a 'breakthrough'?
The government said the partnerships could provide access to enough doses to vaccinate priority groups, including the vulnerable and frontline health and social care workers.
Business secretary Alok Sharma said the agreements would “ensure the UK has the best chance possible of securing a vaccine that protects those most at risk”.
He added: “The hunt to find a vaccine is a truly global endeavour and we are doing everything we can to ensure the British public get access to a safe and effective coronavirus vaccine as soon as possible.”

‘Breakthrough’ inhaled protein treatment
Preliminary results of a randomised clinical trial for a UK drug which could be used as a treatment for COVID-19 show it reduces the number of patients needing intensive care.
Southampton-based Synairgen, the pharmaceutical company developing the drug, which is designed to stimulate the immune system, uses a protein called interferon beta, which the body naturally produces when it gets a viral infection.
The protein is inhaled directly into the lungs of patients with coronavirus using a nebuliser, in the hope that it will stimulate an immune response.
Of hospitalised patients admitted for treatment at nine UK hospitals for COVID-19 infections who inhaled the protein, 79% were found to be less likely to get worse or need ventilation.
Watch video below
Half of the 101 participants of the study were given the drug, called SNG001, while the other half got a placebo.
Synairgen claims patients who took the drug were two to three times more likely to recover to the point where they could carry out everyday activities.
Those receiving the drug also spent less time in hospital – down from an average of nine days to six days.
Chinese vaccine trial found to be ‘safe’
A Chinese phase 2 trial has found a vaccine used on COVID-19 candidates to be “safe” and to induce an immune response, according to new research.
The randomised trial on 508 volunteers, aged around 39 years, follows a phase 1 trial published in May.
The results provide data from a wider group of participants than their phase 1 trial, including a small sub-group of participants aged 55 years and older.
The vaccine in this trial uses a weakened human common cold virus to deliver genetic material to the cells that codes for the SARS-CoV-2 spike protein.
Here is a second COVID-19 vaccine trial, led by Chinese scientists. This recombinant adenovirus type-5 vaccine induces a rapid humoral and cellular immune response within 14 days. No serious adverse reactions. Again, congratulations to Feng-Cai Zhu et al. https://t.co/onf8ZnBy2W
— richard horton (@richardhorton1) July 20, 2020
These cells then produce the spike protein and travel to the lymph nodes, where the immune system creates antibodies that will recognise the spike protein and fight off the coronavirus.
Of the 508 volunteers, 253 received a high dose of the vaccine, 129 received a low dose and 126 received placebo.
Blood samples were taken from participants immediately before the vaccination, 14 days post-vaccination and 28 days post-vaccination to measure antibody responses.
The trial found that 95% of participants in the high dose group and 91% of the recipients in the low dose group showed either T cell or antibody immune responses at day 28 post-vaccination.
But the authors note that it is important to stress that no participants were exposed to COVID-19 after vaccination, so it is not possible for this study to determine whether the vaccine candidate effectively protects against the disease.
Professor Feng-Cai Zhu of the Jiangsu Provincial Center for Disease Control and Prevention said: “The phase 2 trial adds further evidence on safety and immunogenicity in a large population than the phase 1 trial. This is an important step in evaluating this early-stage experimental vaccine and phase 3 trials are now underway.”
Coronavirus: what happened today
Click here to sign up to the latest news and information with our daily Catch-up newsletter
 Yahoo Sports
Yahoo Sports 
